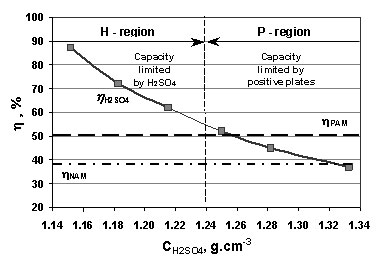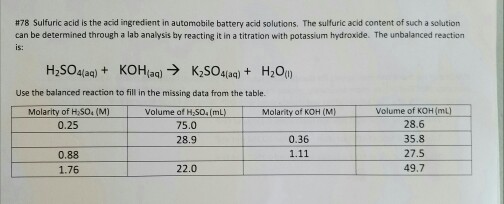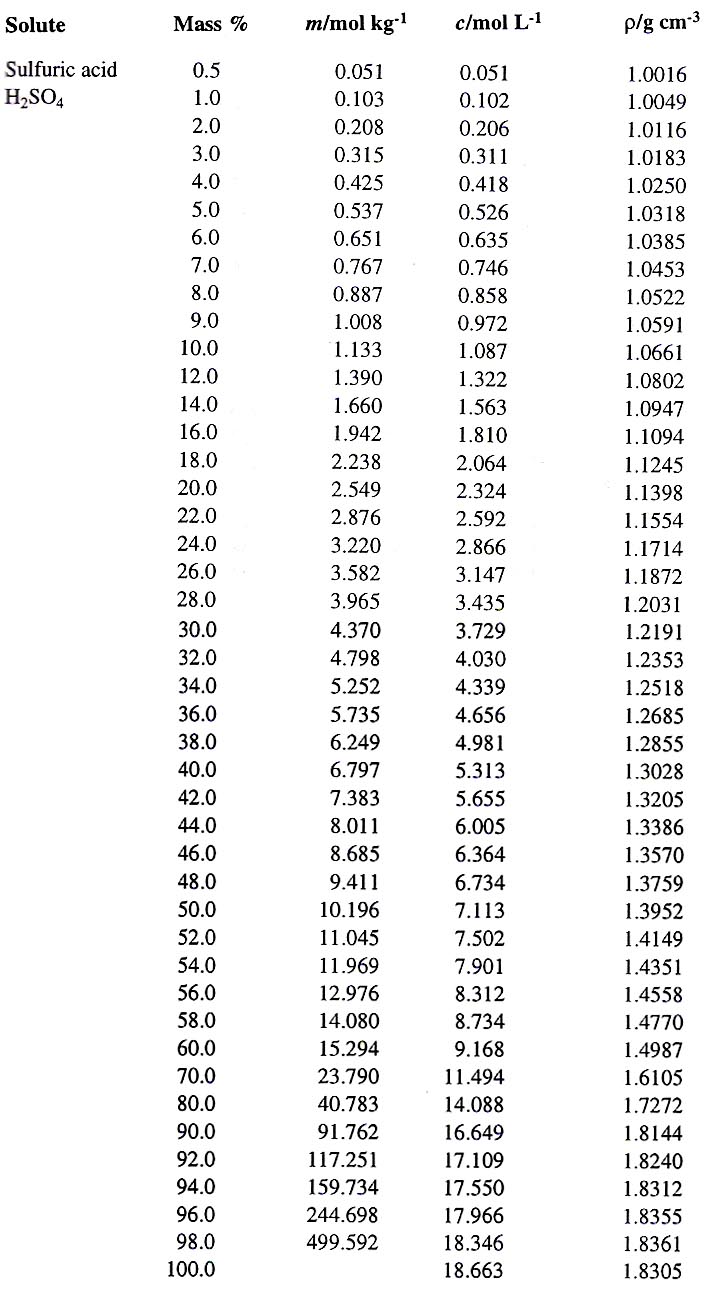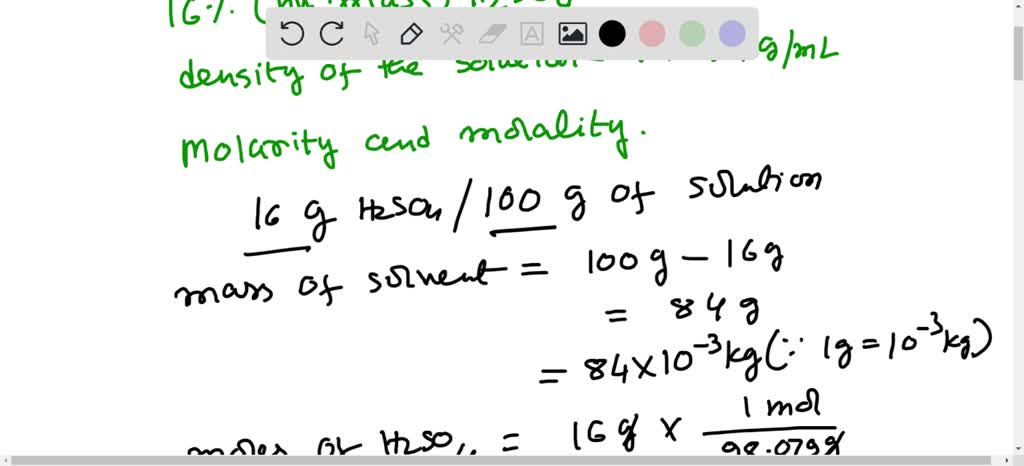
SOLVED: Please help me solve for this, thank you very much. Stay safe Sulfuric acid is also known as battery acid with a Molecular Formula H2SO4 and Molar Mass of 98 grams/mole

Why does the graph of the electrical conductivity of sulfuric acid/water solutions have this knee in the ~85%-~92% range? - Chemistry Stack Exchange

The molarity of sulfuric acid in a fully charged car battery is 5.2 m. when fully discharged the - Brainly.com
Battery acid is 4.27M H2SO4(aq) and has density 1.25 g/ML. What is the molality of H2SO4 in the solution?

SOLVED: called battery acid because It" used In car batterles It Is a very strong acid that can cause Sulfuric add Is often very serious chemical burns upon contact: It can lead

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

Structure of Sulfuric Acid Solutions Using Pair Distribution Function Analysis | The Journal of Physical Chemistry B

Effects of sulfuric acid molarity on HPR with electrolyte solutions... | Download Scientific Diagram

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

What is the molality of a solution containing 200 mg of urea (molar mass 60 g mol^-1 ) dissolved in 40 g of water?

Neutralization Reaction: Determine Molarity of a sulfuric Acid Solution when Neutralized by NaOH - YouTube

Converting mass percent to molarity: the density of a 24.5 mass % solution of sulphuric acid ` - YouTube